Biofilm/Corrosion Whitepaper I
Corrosion
can be defined as the deterioration of material by reaction to its
environment. The corrosion occurs
because of the tendency for most metals to return to their natural state; e.g.,
iron in the presence of water and oxygen (or even moist air) will revert to its
natural state, iron oxide. The development of biofilms and the role they play in corrosion and deposition processes may be the most misunderstood and baffling factor in the management of cooling and other industrial water systems. Ask any water treatment professional about the major attributes of a typical corrosion cell, and you will usually get a reasonable explanation. Ask about biofilms, and you probably wonít get a response. This review is designed to provide a basic understanding of
what biofilms are, the problems they can cause, and what might be done to deal
with them. We
need to begin by answering the basic question, what is a biofilm? Simply
stated, a biofilm consists of microbial cells (algal, fungal, or bacterial) and
the extracellular biopolymer the cells produce. Generally, it is bacterial
biofilms that are most problematic in industrial water systems, since they are
responsible for the blockage and fouling of heat transfer equipment. One should
keep in mind that the more nutrients available in the form of useable organic
carbon, the greater the diversity and numbers of organisms that can be
supported. When dealing with cooling towers and spray ponds, algal biofilms are
also a concern. Not only will algal
biofilms foul distribution decks and tower fill, but algae will also provide
nutrient (organic carbon) that will help support the growth of bacteria and
fungi. Algae does not require organic
carbon for growth but instead utilizes CO2 and the energy provided
by the sun to manufacture carbohydrates.
So, a cooling water system that begins with little or no organic carbon
can generate a supply through the growth and dispersal of algal cells. The
second question that needs to be answered is, why does biofilm develop in some
areas rather that others? In many instances, the piping and other
apparatus serviced by a cooling tower is comprised of various and dissimilar
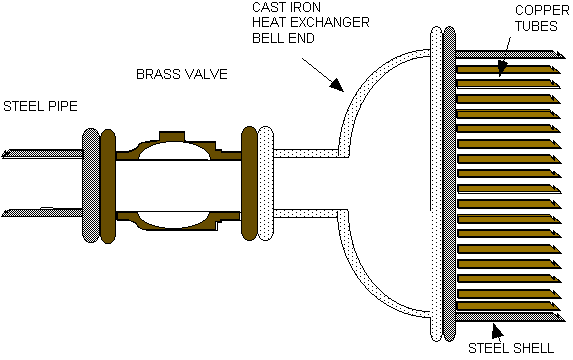
materials. Figure 1 below is typical of
most applications. Dissimilar materials in piping and process
systems create galvanic corrosion which is
Fig. 1 - Dissimilar metals the
source of the food, energy, and oxygen needed for the multiplication of the
microorganisms that generate the biofilm as a home. The bacteria which is the heart of the biofilm formations needs
basically the same things that all living creatures need: oxygen, food, energy, and a home (shelter from predators [biocides]). Although there is no absolute relationship
between existing galvanic action, which generates a food, energy, and oxygen
supply, and the development of the microorganisms that create biofilm homes
(shelter from predators [biocides]), the presence of a ready food, energy, and
oxygen source certainly makes microbial development stress free for the
ìcrittersî. However, one cannot
categorically state ìif electrolytic corrosion is allowed to begin-then
microbial corrosion will immediately followî, but a prudent facilities manager
will not rest until the electrolytic corrosion has been neutralized.
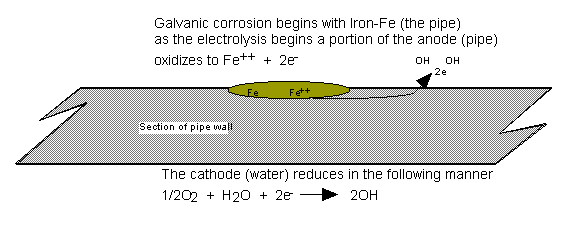
As
Fig. 2 illustrates, galvanic activity generates iron oxide and two free
electrons. Due to the impurities in
steel and also due to mechanical stress, steel does not have a uniform standard
reduction potential across its surface.
The result is that certain areas on the surface are more prone to
pitting and corrosion than others. When dissimilar metals are in electrical or
physical contact (or through an electrolyte, i.e. water), galvanic corrosion
can take place.
The process is similar to a DC
cell in that the more active metal (less Nobel) becomes the anode and corrodes,
and the less active metal behaves like a cathode and is protected. Corrosion is
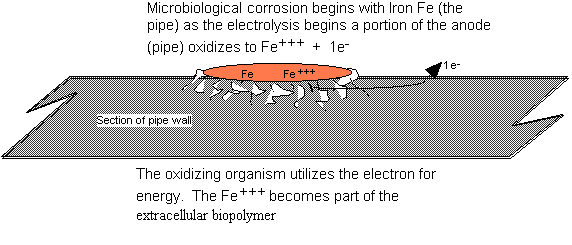
often defined as the deterioration of material by reaction to its environment. In microbiological corrosion (as
shown in Fig 3), the iron reduces to Fe+++ + 1e- (unlike galvanic which is Fe++ + 2e-)
and the electron is the energy for growth; the oxide is converted to oxygen for
the bacteria. The secretion of the iron
may be in any one of several forms, but all of the forms become housing- or
sacrificial shelter- for the generating organism. Microorganisms can influence
corrosion in a variety of ways. Formation of localized differential cells, the
production of mineral and organic acids, ammonia production, and sulfate
reduction are just a few of the mechanisms by which bacteria, fungi, and algae
can influence corrosion. The formation
of localized cells is the primary mechanism of corrosion caused by iron
oxidizing bacteria. When iron and manganese
oxidizing organisms colonize a surface, they immediately begin to oxidize
available reduced forms of these elements and produce a deposit. As the bacteria colony becomes
encrusted with iron (or manganese) oxide, a differential oxygen concentration
cell will develop, and the corrosion process begins. The porous encrustation (tubercle) may potentially become an
autocatalytic corrosion cell or may provide an environment suitable for the
growth of sulfate-reducing bacteria. Corrosion may also develop when
localized cells are formed, due simply to biofilms developing on metal surfaces
(when no galvanic corrosion had ever existed).
The oxidation of iron or manganese is not always a requirement for the
development of a localized corrosion cell. There are numerous factors that
can contribute to localized corrosion on metal surfaces. The production of ammonia by the reduction
of nitrates may lead to severe localized losses in copper based metallurgy. Inorganic acids, such as sulfuric acid
produced by Thiobacillus sp., can
also have detrimental surface effects.
As biofilms develop, they will eventually achieve a thickness at which
oxygen concentration is either very low or completely excluded. At a thickness of just 200 microns, the
oxygen concentration within the biofilm is reduced to near zero ppm. When this
occurs, facultative and obligate anaerobes can flourish. The import is that anaerobes must generate
their own oxygen which means that corrosion is, and must be for sustenance and
growth of the anaerobes, rampant.
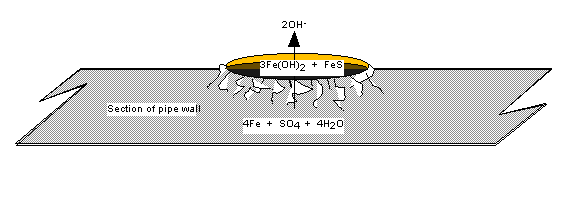
Anaerobic sulfate-reducing
bacteria, such as Desulfovibrio sp.,
is the bacteria most often considered when discussing microbial corrosion. This
organism will seek out and colonize areas deficient in oxygen, such as those
found within porous corrosion tubercles, within biofilms, and under debris. This bacteria is responsible for severe
metal loss in industrial water systems.
This type of corrosion is easily recognizable from the characteristic
sulfide by-product present within the corrosion cell. Sulfate reducing bacteria primarily cause corrosion by utilizing
the molecular hydrogen produced at the cathode, thereby depolarizing it. Systems that are sulfate limited will have
less of a tendency to be attacked by SRB.
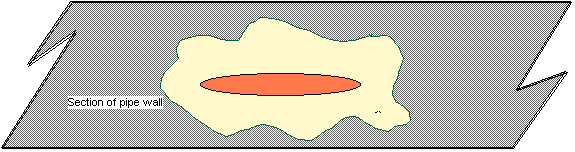
Fig. 5 - Fimbriae & multiple fimbriae
floating through pipe Microorganisms can be found in
both the bulk water and on the surfaces of industrial water systems. Bacteria
attach to surfaces by proteinaceous appendages referred to as fimbriae (Fig.
5).
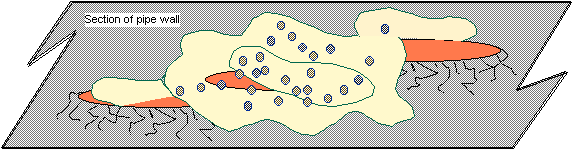
Fig. 6 - Attachment of fimbriae to a surface Once a number of fimbriae have
"glued" the cell to the surface (Fig. 6 above), it makes detachment
of the organism very difficult. One
reason bacteria prefer to attach to surfaces is the organic molecules adsorbed
there provide nutrients (oxygen & energy).
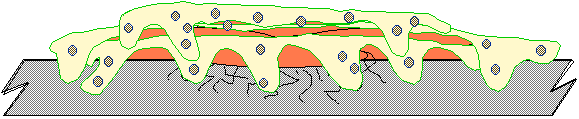
Fig. 7 - Attached fimbriae is surrounded by
extracellular biopolymer Once attached, the organisms begin
to produce material termed extracellular biopolymer or "slime" for
short. The amount of biopolymer
produced can exceed the mass of the bacterial cell by a factor of 100 or
more. The extracellular polymer that is
produced provides a more suitable protective environment for the survival of
the organism. It
can be seen that the growth of bacteria on surfaces in cooling and process
water systems can lead to significant deposit and corrosion problems. Once this
is understood, then the importance of controlling biofilms becomes quite clear.
Too often microbiological control efforts focus only on planktonic counts, that
is to say the number of bacteria in the bulk water. While useful data can be gathered from monitoring daily bacterial
counts, monthly or weekly counts are simply time wasted and have little
meaningful use if one is attempting to assess the presence and quantity of the
microorganisms that produce biofilm.
The microorganisms that produce extracellular polymer and hide under and
within the slime are not borne freely through the bulk water to be counted as
part of plankton count. Therefore,
Planktonic counts do
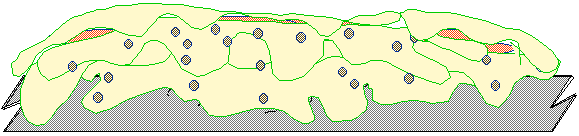
Fig. 8 - The microorganism has begun to cover
itself for protection not
have any correlation to the amount of biofilm that may be present. In addition,
planktonic organisms are not generally responsible for deposit and corrosion
problems. There are a few exceptions, generally within closed loop systems,
where planktonic organisms may degrade corrosion inhibitors, produce high
levels of H2S, or reduce the pH. Systems with low planktonic counts
may have a significant biofilm problem and vice versa. Therefore, efforts
should focus on general biofilm detection and control.
Fig. 9 - Multiple colonies develop as oxygen,
energy and nutrients permit Once
bacteria begin to colonize surfaces and produce biofilms, maintenance and
operational problems arise, including reduction of flow, reduction in heat
transfer rates, fouling, corrosion, and scale.
When biofilms develop in low flow areas, such as cooling tower film fill
or piping ìdead legsî, they may go unnoticed for months or years, since they
will not initially interfere with flow or evaporative efficiency. After time, the biofilm becomes more
complex, accompanied by robust filamentous development. The matrix provided will accumulate debris
that will initially impede flow and eventually completely block flow at heat
exchangers and cooling jackets. Any
sudden increase in nutrient supply in a previously nutrient-limited environment
will send bacterial populations into an accelerated logarithmic growth phase
with rapid accumulation of biofilm. All
of the biofilms that develop, if left uncontrolled, will eventually interfere
with heat transfer efficiency.
Fig. 10 - In time the original colony has
insulated itself Biofilm structure is often
imagined as a coating of microbial cells and the secreted biopolymer spread
evenly across a surface. In reality,
biofilm structure is much more complex, including patchy and highly channelized
development, which allows nutrient-bearing waters to flow through the
extracellular polymer providing the microorganism with a ready nutrient
supply. If allowed to multiply
unrestrained, the organism eventually insulates itself in the shelter of slime
it constructs and becomes almost impossible to destroy. Algal biofilms will foul cooling
tower distribution decks, tower fill, and basins. When excessive algal biofilms develop, portions eventually break
loose and transport to other parts of the system, causing blockage and
providing nutrients for accelerated bacterial and fungal growth. Biofilms can cause fouling of filtration and
ion exchange equipment . As seen below,
biofilm is the most damaging development that can occur in a heat exchange
environment. Thermal
conductivity comparison of deposit-forming compounds and biofilm
A
lower number indicates a greater resistance to heat transfer. Coatings and deposits in the form of biofilm and biofilm with
entrapped suspended debris are foulants that most of us can comprehend, but
biofilms often lead to the additional formation of mineral scales as well. Calcium ions can attach to the biofilm by
the attraction of carboxylate functional groups on the polysaccharides, or
calcites may simply become trapped in the matrix. In fact, divalent cations, such as calcium and magnesium, are
integral to the formation of certain extracellular polysaccharide gels. Many
of the microorganisms develop more rapidly in elevated temperatures and
therefore seek out heat exchanger entrances and surfaces, which means the
calcium ions are fixed in place at the heat transfer surface. This further
complicates and impedes the heat exchange efficiencies because the ions are
then positioned to react with carbonate or phosphate anions as they become
available, which provides additional nucleation or crystal growth sites in and
on the biofilm. ______________________________________________________ Biofilms can be controlled through
the use of microbicides, biodispersants, and by limiting nutrient.
Microbiocides, both oxidizing and nonoxidizing, can be effective in overall
biofilm control when applied properly. The oxidizing microbicides, such as chlorine,
bromine, chlorine dioxide, and ozone, can be extremely effective in destroying
both the extracellular polysaccharide and the bacterial cells. When using
oxidizing microbicides, one must be sure to obtain a sufficient residual for a
long enough duration to effectively oxidize the biofilm. Unfortunately, there
are those who are overly concerned with the corrosive nature of the oxidizing
microbicides and fail to apply the needed residual oxidant required to control
biofilm. Low residual oxidant levels may significantly reduce planktonic counts
but may not be sufficient to control biofilm. The level of oxidant and duration
required will vary from system to system. It is generally more effective to
maintain a higher residual for a duration of several hours than it is to
continuously maintain a low residual. Continuous low-level feed may not achieve
an oxidant level sufficient to oxidize the polysaccharide and expose the
bacteria to the oxidant. Another misconception is with the
use of chlorine at alkaline pH (> 8.0). It is often thought that chlorine is
ineffective for controlling microorganisms at elevated pH. This is only half
true. Certainly, the hypohalous acid form of chlorine (HOCl) is more effective
at killing cells than the hypohalite form (OCl-). However, the hypohalite is
actually very effective at oxidizing the extracellular polysaccharide and the
proteinaceous attachment structures. Therefore, the use of chlorine in alkaline
cooling waters can still be extremely effective. This is especially true when
combining chlorine with bromine or with a compatible nonoxidizing microbiocide
such as a polyquat. When this is done, you achieve both oxidation of the
extracellular material and sufficient kill of the microorganism. Nonoxidizing microbicides are also
effective in controlling biofilm. Effective control is greatly dependent on
frequency of addition, level of feed, and resistance of the incumbent
population to the product being fed. Control cannot generally be achieved by
once-a-week additions as is common in "full service" applications.
Typical application for effective control may include a slug addition of
product 2 to 5 times a week. As with oxidizing microbicides, frequency and
dosage will depend on the system conditions. It is generally most effective to
alternate nonoxidizing microbicides at every addition to ensure broad spectrum
control. Most nonoxidizing microbicides will have little effect in destroying
the extracellular polysaccharide found in the biofilm. However, many
microbicides may be able to penetrate and kill bacteria found within the
biofilm. Combining the use of nonoxidizing and oxidizing microbicides is a very
effective means of controlling biofilm. When using a nonoxidizing
microbiocide in conjunction with an oxidizing agent, there should be no
residual oxidant present in the system at the time of addition. Sufficient time
should be allowed for the nonoxidizing microbiocide to work before resuming
oxidant feed unless an oxidant compatible microbiocide is being used ( i.e.,
polyquat). Biofilm control programs can be
made more effective through the utilization of a biopenetrant/dispersant
product. Products that penetrate and loosen the biopolymer matrix will not only
help to slough the biofilm but will also expose the microorganisms to the
effects of the microbiocide. These products are especially effective when
dealing with systems that have a high TOC loading and a tendency to foul. These products are typically fed
in slug additions prior to microbiocide feed. Low-level continuous feed may not
be as effective, since it often takes a certain threshold amount to produce the
desired effect. Recent developments in biodispersant technology is making this
approach more effective and popular than ever before. Enzyme technologies that will
break down the extracellular polysaccharides and degrade bacterial attachment
structures (fimbriae) are currently being developed and patented. These
technologies, although expensive, may provide biofilm control where
microbiocide use is environmentally restricted or provide a means of quickly
restoring fouled cooling water systems to a clean, efficient operable state.
The importance of biofilm control must not be taken lightly. It is the
fundamental basis for controlling a high percentage of deposition and corrosion
problems in process and cooling water systems. Once these fundamentals are
understood, effective treatment strategies can be developed. The growth of bacteria and
formation of biofilms may also result in another problem, that of corrosion.
Microbiological corrosion may be defined as corrosion that is influenced by the
growth of microorganisms, either directly or indirectly. To understand
microbiological corrosion or MIC for short, it helps to have a basic
understanding of corrosion chemistry. This document is not intended to provide
that information, but any water treatment training manual will. In essence,
corrosion occurs on a metal surface due to some inherent or environmental
difference between one area on that surface and another. These differences will
create anodic and cathodic areas, setting up a basic corrosion cell (Fig. 7).
The anode is the area at which metal is lost. The electrons given up by the
metal flow to the cathode to be consumed in a reduction reaction. Microbiological
corrosion is electrochemical corrosion where in some manner the presence of the
microorganisms is having some influence in the creation or acceleration of
corrosion processes. |